|
Main Topics > Quantum Theory and the Uncertainty Principle > Quanta and Wave-Particle DualityThe earliest steps in the development of quantum physics arose from the investigation into something as mundane as why metal glows red when hot. The great German physicist Max Planck had been studying the problem of black body radiation in the late 1890s. The “problem” Planck was dealing with was the observation that the greatest amount of energy being radiated from a black body (or any perfect absorber) actually falls near the middle of the electromagnetic spectrum, rather than in the ultraviolet region as classical theory suggested. While Planck’s initial black body radiation law described the experimentally observed black body spectrum quite well, it was not perfect, and it was Planck’s genius to realize that the only way the law could work perfectly was to incorporate the supposition that electromagnetic energy could be emitted only in “quantized” form (i.e. restricted to discrete values rather than to a continuous set of values). In 1900, he proposed that light and other electromagnetic waves were emitted in discrete packets of energy, which he called "quanta", which can only take on certain discrete values (multiples of a certain constant, which now bears the name the “Planck constant”). He concluded that the energy radiated from a black body could only be a multiple of an elementary unit, E, where E = hv (where h is the Planck constant, and v is the frequency of the radiation). In effect, Planck showed that the very structure of nature is discontinuous, in the same way as the population of a city, for example, can only change in discrete increments (i.e. whole number of people). Although, quantization was a purely formal assumption in Planck’s work at this time, and he never fully understood its radical implications (that had to await Albert Einstein’s interpretations in 1905), it has come to be regarded as the first essential stepping stone in the development of quantum theory, and the greatest intellectual accomplishment of Planck's career, for which he was awarded the Nobel Prize in Physics in 1918.
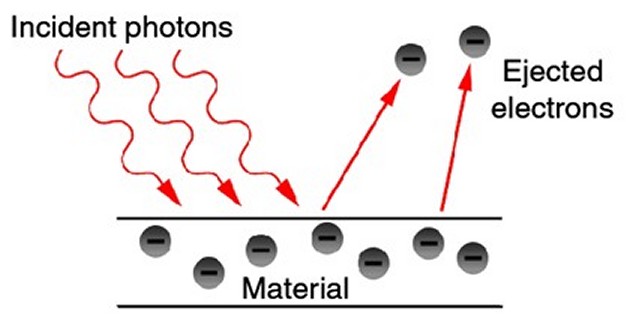
Building on this earlier research by Planck and by Philippe Lenard, Einstein became, in 1905, the first person to clearly realize that light was made up of photons. He saw it as the only way to make sense of the so-called "photoelectric effect" (the phenomenon whereby certain metals, when exposed to light, eject electrons). Einstein found that, no matter how bright the light shone on the metal, only light above a certain frequency caused electrons to be given off. Above that point, as the frequency of the light is increased, the energy of the electrons given off also increased. Furthermore, he noted that all the electrons were emitted instantaneously, with no delay whatsoever, which could not happen if the light was a wave sweeping over the metal, but only if the electron emissions were caused by individual particles of light. Einstein, therefore, extended Planck’s discovery by theorizing that energy itself (not just the process of energy absorption and emission) is quantized. Light, he concluded, must consist of tiny bullet-like particles, now known as photons. In fact, it was for this work on the photoelectric effect in 1905 that Einstein was awarded the 1921 Nobel Prize in Physics, not for his better-known work (in the same year) on the Special Theory of Relativity. In 1913, the Danish physicist Niels Bohr further built on Planck’s insights and on the recent discoveries of J. J. Thomson and Ernest Rutherford about the structure of atoms. Bohr introduced the idea that electrons can only orbit an atom's nucleus at certain discrete distances (or "shells"), orbits that are different for different elements. This happens because electrons are also waves of specific frequencies, and the waves only fit (without interfering with themselves or canceling each other out) on orbits of certain sizes. Electrons closer to the nucleus have lower energy than those further away (even though they are traveling faster).
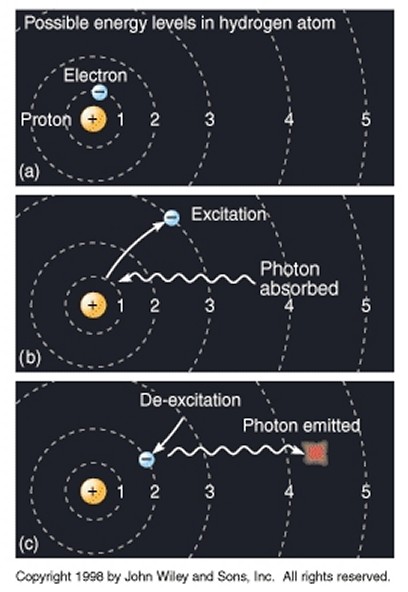
However, although an electron can only exist in certain discrete energy levels (or "quantum states"), it can move from one energy level to another. For example, if an atom is heated or forced to collide, the energy imparted can cause an electron to move to a higher energy level (we say that the electron is "excited"). Bohr noted that it did not gradually pass through a continuum of energy levels in between, but rather there was a "quantum leap" or “quantum jump”, and the electron instantly leaped from one energy level to the next. A useful analogy is that of climbing a set of stairs, where it is possible to stand on any given step, but not somewhere in between two steps. He also discovered that when an electron drops from a higher energy orbit to a lower one - which it will do whenever there is a lower energy state available for it to occupy - it emits in the process a photon (an individual quantum, or packet, of electromagnetic radiation) with energy exactly equal to the difference between the energy levels of the two orbits. Conversely, if light with the right energy strikes an atom, then its electrons will be excited and rise to a higher energy state, and the light will be absorbed. This phenomenon is essentially why a heated object glows: the heat causes electrons to jump into excited states; then, when they drop back down to the "ground" state, the atom gives off photons of light. It is also the basis for the invention of the laser: in a nutshell, energy is pumped into atoms, thereby exciting them, and then, when the electrons drop down in energy, the photons emitted are collected and focussed. It had been observed for some time (dating back to Anders Jonas Ångström in 1853) that, as atoms were heated, light was emitted not as a diffuse, blurred smear but in distinct and separate bands of color (i.e. different wavelengths), with each element producing its own unique spectral pattern, its own distinct "spectral fingerprint", but it had always been beyond the explanatory powers of classical physics. Bohr's revelation, that an electron jumps from one distinct state to another, neatly explained why the light was emitted in distinct bands of color, as electrons with specific energy levels within different elements changed their quantum states. For example, an electron moving from the third orbit of an atom to the second orbit emits red light, from fourth to second creates blue-green light, from fifth to second violet light, etc, all corroborated by Bohr's model. This arrangement of the electrons within atoms also has some very useful practical applications. Because of the very structured and regular arrangement of atoms in solids, the energy levels of electrons within constituent atoms combine to form continuous energy bands (known as valence bands) separated by band gaps. The band structure of a material determines several characteristics, in particular the material's electronic and optical properties (e.g. some materials have very close, or even overlapping, bands so that electrons can easily move between them, which makes them good conductors of electricity; other materials have very large band gaps which makes them good insulators; etc). So, the early stepping stones towards a fundamentally new type of physics (which was to become known as quantum theory or quantum mechanics) were gradually falling into place, and it was becoming clear that an essential element of it was the conception of light (and indeed all radiation and all matter) as composed of discrete quanta or particles.
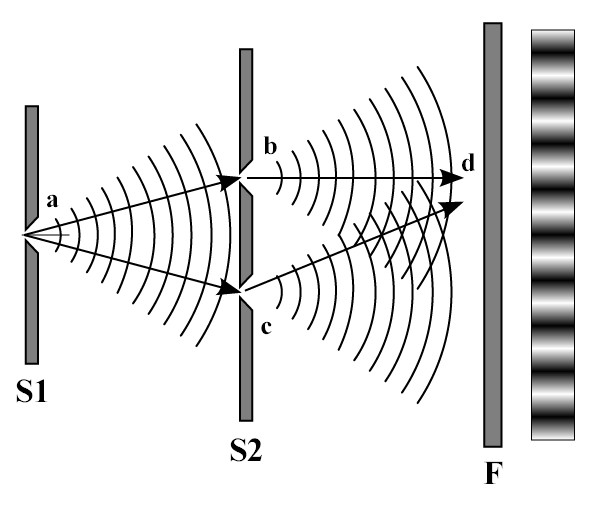
However, it had already been demonstrated beyond doubt that light was in fact a wave. Thomas Young’s experiments with his “double-slit” apparatus at the beginning of the 19th Century had shown that light caused interference, a characteristic property of waves, and he was even able to determine its wavelength, which he established was less than a thousandth of a millimeter. This had seemed at the time to settle forever the dispute which had been raging since the 17th Century between those (such as Christiaan Huygens) who favored a wave theory of light and others (such as Sir Isaac Newton) who favored a corpuscular or particle theory of light. The developments by Einstein and Bohr in the early decades of the 20th Century, therefore, meant that physicists had to come to terms with the idea that light was both a wave AND a particle, and that sometimes it behaved like a wave and sometimes it behaved like a particle, an idea which became known as wave-particle duality. In an absolute sense, then, light is actually neither a particle nor a wave, but only exhibits wave or particle properties, depending on the experiment being performed. Such an idea, however, was totally incompatible with all physics that had gone before, and particularly with the whole edifice of Maxwell’s theory of electromagnetic waves which had become by that time the orthodoxy of classical physics. It was also impossible to visualize and totally counter-intuitive at first glance. Perhaps wave-particle duality may be most easily understood by analogy: consider, for example, that a novel is both a story and a collection of individual words; or that the mind consists simultaneously of both thoughts and a series of electrical impulses. But there was more to come. In 1923, Arthur Compton’s famous “Compton scattering” experiment showed how x-rays (generally understood as waves of electromagnetic radiation) can be observed to bounce off electrons, thus exhibiting particle-like properties, just like billiard balls impacting with other billiard balls. He also showed how this particle-like characteristic of electromagnetic radiation could be measured by its frequencies, previously considered a characteristic property only of waves. Furthermore, in 1924, the French physicist Louis de Broglie showed that wave-particle duality was not merely an aberrant behavior of light, but rather was a fundamental principle exhibited by both radiation and ALL particles of matter. According to de Broglie’s findings, then, at least in theory, everything (a baseball, a car, even a person) has a wavelength, although their wavelengths are so small as to be not noticeable. Just as Planck and Einstein had shown that waves can have particle-like characteristics, de Broglie showed that particles can have wave-like characteristics. These counter-intuitive claims were backed up by double-slit experiments using electrons instead of light, in which the same kind of wave-like interference patterns were demonstrated as in Thomas Young's early experiments with light. In a strange twist of fate, George Thompson received the 1937 Nobel Prize in Physics for definitively proving the wave properties of the electron, just as his father had won the 1906 Prize for his discovery of the electron as a particle. Thus, it became clear that a particle like an electron (or even an atom) could in some way interfere with itself, and was in some sense "spread out", or at least was able to be in many places at once. It should be noted, though, that this is not to say that an atom can spread itself out in a broad beam of some sort: the wave we are talking about is a wave of information, of what can be known about the atom, a probability wave (which we will discuss in more detail in the next section). Essentially, the wave is not the particle itself but a measure of the probability attached to its particle nature.
|
Back to Top of Page
Introduction | Main Topics | Important Dates and Discoveries | Important Scientists | Cosmological Theories | The Universe By Numbers | Glossary of Terms | A few random facts | Blog | Gravitational Lensing Animation | Angular Momentum Calculator | Big Bang Timeline
NASA Apps - iOS | Android
The articles on this site are © 2009-.
If you quote this material please be courteous and provide a link.
Citations | Sources | Privacy Policy